
📚 Strengthening Our Expertise: Product-Specific Guidance
At Pharmexon, we believe in fostering a culture of continuous learning to stay ahead in a fast-paced regulatory landscape. Parvinder Punia recently led a focused
We are a lifesciences consultancy company focused on expert advice, encompassing pre-development pathways, ongoing support during development, assistance in development of the compliant documentation and all the activities required for successful registration and maintenance of medicines and devises.
We are a lifesciences consultancy company. Focused on expert advice, encompassing pre-development pathways, ongoing support during development, assistance in development of the compliant documentation and all the activities required for successful registration and maintenance of medicines and devises
We combine the strength of our of expertise, experience and creative strategies to support the pharmaceutical companies to deliver life changing therapies faster to the market. also understand how to consult for Industry.
This unique vision means that the experts are able to assess the technical complexities, scientific challenges and regulatory landscape with full accountability to the end business requirements. One of the main challenges our experts have experienced is to make Business Development see eye to eye with Regulatory. The two elements are not mutually exclusive and all elements are considered as part of any strategy.
Pharmexon Consulting is able to see Regulatory Affairs with a 3¬dimensional view. What is unique about our consultancy is that our Key Experts have worked for Health Agencies, Corporate Pharmaceuticals and have consulted in small to large service company.
We strive to be the preferred firm for companies looking to prioritize the well-being of patients and place them at the heart of their strategic decisions. By doing so, we aim to make a significant and positive impact on the healthcare landscape.
A team of young, hard-working, open-minded and friendly colleagues
An exciting and challenging job in an innovative, flexible, and growing company
Initiation and trainings in Pharmacovigilance field reflecting the latest European requirements


At Pharmexon, we believe in fostering a culture of continuous learning to stay ahead in a fast-paced regulatory landscape. Parvinder Punia recently led a focused

The Pharmaceutical Industry is incredibly dynamic, with constant updates occurring all through various fields encompassing the industry. Pharmexon has gathered Regulatory Intelligence from September 3rd
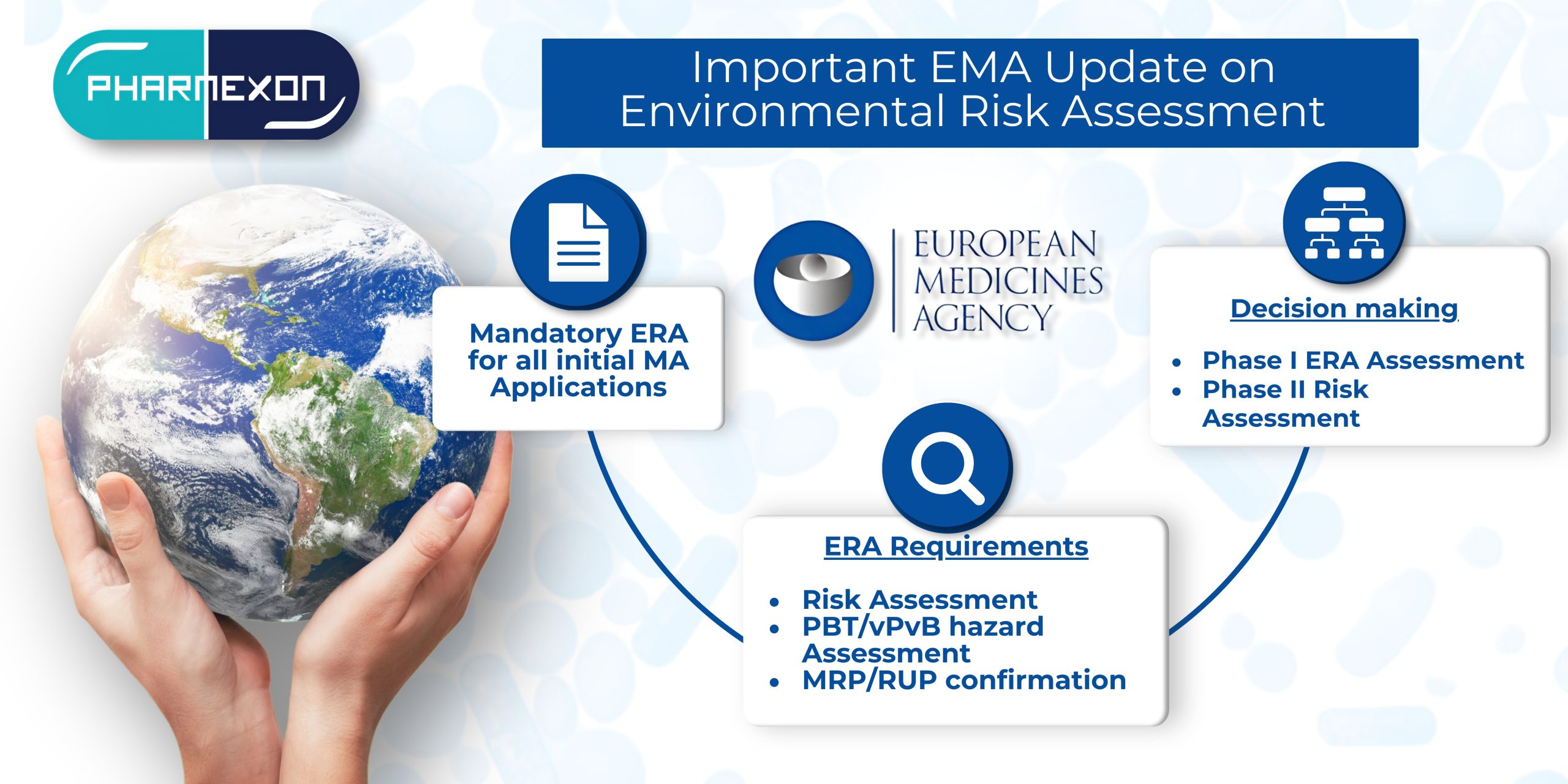
This fall a significant EMA update of guideline on Environmental Risk Assessment (ERA) for Medicinal Products for human use took effect! 🔍 Key Highlights: 🌍
+420 602 378 935
Pharmexon Consulting s.r.o
Štěpánská 65, Prague 1
11000 Prague, Czechia